Summary
Glycation refers to an abnormal, unwanted attachment of sugar molecules to different tissues and structures, leading to potential damage and inflammation, whereas glycosylation is a normal, desirable process that is essential for healthy cellular and physiological function.
Definition of Glycation
Glycation is the non-enzymatic attachment of monosaccharides (simple sugar molecules) to proteins, lipids, and nucleic acids, resulting in the formation of molecules known as advanced glycation end-products (AGEs).
In simpler terms, glycation is an abnormal process where sugar molecules attach themselves to proteins, lipids, or nucleic acids without the cell needing or regulating this attachment. This process occurs without the involvement of enzymes. As a result, these glycated molecules—called AGEs—can accumulate in cells and tissues, leading to dysfunction and inflammation.
Glycation vs. Glycosylation: Understanding the Difference
Glycosylation is a normal, physiological process where sugar molecules are enzymatically attached to proteins or lipids to serve various essential cellular functions. For example, endothelial cells, which line our blood vessels, have a surface structure called the glycocalyx. This glycocalyx is a glycosylated layer that coats the cell surface, helping make it slippery. This slipperiness is crucial as it allows blood cells and proteins to glide over the endothelial surface without sticking, thereby maintaining smooth blood flow. The sugars in the glycocalyx also bind water, further contributing to this slippery property.
In summary, glycation refers to an abnormal, unwanted attachment of sugar molecules to different tissues and structures, leading to potential damage and inflammation, whereas glycosylation is a normal, desirable process that is essential for healthy cellular and physiological function.
Importance of Glycation in Health and Disease
1. Diabetes Mellitus
Glycation is most commonly associated with diabetes mellitus. In diabetic patients, elevated blood glucose levels result in a higher concentration of glucose outside cells. This excess glucose leads to glycation across virtually all tissues, causing tissue dysfunction and inflammation.
You may wonder why inflammation is frequently mentioned alongside tissue dysfunction. The reason is that when tissues or cells become dysfunctional, they often release stress signals in the form of cytokines. These cytokines then trigger inflammatory responses. The mechanisms behind this interplay between dysfunction and inflammation are complex and would require an entire book to explain, so we will keep our focus on glycation’s role in diabetes for now.
Returning to glycation in diabetic patients, this process contributes to various complications, such as neuropathy (nerve damage), retinopathy (damage to the retina of the eyes), and nephropathy (kidney damage). In all these cases, glycation-induced tissue damage and inflammation play a critical role.
2.Cardiovascular System Diseases
Advanced glycation end-products (AGEs) contribute to inflammation of blood vessel walls, which can lead to several cardiovascular conditions. This inflammatory response promotes the development of atherosclerosis (the buildup of plaques in the arteries), increasing the risk of heart attacks, heart failure, and strokes.
3. Neurodegenerative Diseases
Glycation has been implicated in several neurodegenerative diseases. For example, in Alzheimer’s disease and Parkinson’s disease, the accumulation of glycated proteins may contribute to neuronal damage and cognitive decline. This connection suggests that glycation could play a critical role in the progression of dementia and other neurodegenerative conditions.
4. Other Conditions
Glycation is also associated with several other health issues, including accelerated aging, systemic inflammation, certain cancers, and obesity. The accumulation of AGEs can disrupt normal cellular functions and promote an inflammatory environment, potentially contributing to the development or worsening of these conditions.
Let’s now understand glycation in further detail. The purpose of this section is to provide you with knowledge that you can use to adjust your lifestyle, food habits, and medicines (if needed) to keep glycation controlled.
Basics of Glycation
What are sugars and proteins?
Sugars are carbohydrates that serve as a primary energy source for our bodies. They come in simple forms like glucose and fructose, or complex forms like starches. Proteins are large, complex molecules made up of amino acids. They play crucial roles in our body’s structure, function, and regulation of tissues and organs.
I will not go into the details for lipids and nucleic acids. However, the process and fate of glycation is similar for these molecules as is for proteins.
Let’s now review the process of glycation.
A Look Into the Glycation Process
Glycation occurs in our bodies at both normal and elevated glucose levels. For example, Hemoglobin A1c (HbA1c) levels measure the glycation of hemoglobin molecules, reflecting the percentage of hemoglobin that is glycated. You might already know that an HbA1c level of less than 5.7% is considered normal. But if glycation is harmful, why isn’t 0% glycated hemoglobin the goal?
The answer lies in how our bodies regulate glucose. Our bodies are designed to maintain glucose levels around 80 mg/dL, even when we do not consume carbohydrates. If you stop all sugar intake, your liver will perform gluconeogenesis (new glucose formation) to maintain normal glucose levels. This glucose is essential for the function of glucose-dependent tissues, such as the brain, proximal convoluted tubules in the kidneys, red blood cells, retina, and peripheral nerves. The glucose at these levels will naturally cause some degree of glycation, which is why an HbA1c of less than 5.7% is considered normal.
However, when glucose levels in the blood and interstitial tissues (the spaces between cells) exceed this range, more glycation occurs potentially leading to organ dysfunction and inflammation. With this context in mind, let’s examine the glycation process more closely.
An Overview of the Molecular Mechanisms of Glycation
Glucose present in the bloodstream, interstitial tissues, and within cells comes into contact with proteins, lipids, and nucleic acids. For simplicity, let’s focus on how glycation affects proteins.
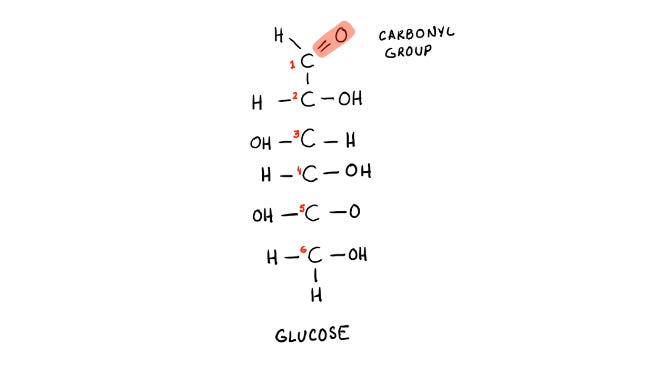
Formation of a Schiff Base: When a simple sugar molecule comes into contact with a protein, the carbonyl group of the sugar can spontaneously bond with the primary amino group of the protein. This initial, weak attachment forms what is known as a Schiff base. At this stage, the glucose is only weakly bound to the protein and can be removed if glucose levels drop—for example, if you engage in physical activity like walking after eating.
Formation of Amadori Products: If glucose concentrations remain high, the Schiff base undergoes a rearrangement to form a slightly more stable structure known as Amadori products. This process involves further organization and mild dehydration, making the glucose more firmly attached to the protein.
Advanced Glycation End Products (AGEs): If elevated glucose levels persist, the Amadori products continue to undergo a series of complex chemical reactions, such as oxidation and further dehydration. This process leads to the formation of highly stable and irreversible structures called advanced glycation end-products (AGEs).
AGEs can accumulate in various tissues, contributing to inflammation, oxidative stress, and tissue dysfunction, which can lead to several health complications over time.
Glucose molecule. Notice the carbonyl group highlighted in red. This group will attack a protein, lipid, or nucleic acid with spontaneous bonding.
Amino Acid (proteins are made up of one or more amino acids in a string.) Note the primary amino group. This group will bind to the carbonyl group of the glucose. Also note the short hand for the protein at the bottom of the image (P-NH2)
Process of glycation i.e. connecting amino acids with sugars. Here the carbonyl group of a glucose binds with the amino group of the protein to form a glycated protein.
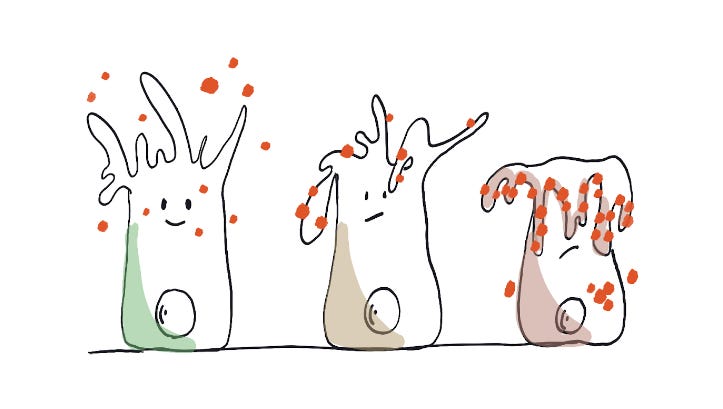
Tissue dysfunction and inflammation as a result of glycation. A green healthy respiratory epithelial cell has its cilia free to beat. Red glucoses are abundantly present around it. These molecules start glycating the cell surface including the cilia. On the right side of the diagram is a fully glycated epithelial cell with its dysfunctional cilia. This person will develop more respiratory congestion and infections.
Glycation can lead to significant organ dysfunction and inflammation by affecting various tissues throughout the body. A non exhaustive list of such damage follows:
Tissues and Associated Pathologies Affected by Glycation:
Blood Vessels:
Atherosclerosis
Hypertension
Peripheral artery disease
Nerves:
Peripheral neuropathy
Autonomic neuropathy
Eyes:
Diabetic retinopathy
Cataracts
Kidneys:
Diabetic nephropathy
Chronic kidney disease
Lungs:
Chronic obstructive pulmonary disease (COPD)
Asthma
Heart:
Coronary artery disease
Heart failure
Skin:
Accelerated skin aging
Impaired wound healing
Musculoskeletal System:
Osteoarthritis
Reduced joint mobility
Immune System:
Immunodeficiency
Autoimmune disorders
Pancreas:
Type 2 diabetes mellitus
Beta-cell dysfunction
Liver:
Non-alcoholic fatty liver disease (NAFLD)
Non-alcoholic steatohepatitis (NASH)
Brain:
Alzheimer’s disease
Cognitive decline
Gastrointestinal Tract:
Gastroesophageal reflux disease (GERD)
Gastroparesis
Let’s take two key examples of how glycation contributes to such damage.
1. Respiratory Epithelial Dysfunction and Inflammation
The respiratory epithelium, which lines the airways, acts as a critical barrier to protect the lungs from environmental pollutants, pathogens, and allergens. Glycation can impair the function of these epithelial cells by altering the proteins and lipids that form the structural and functional components of the cell membranes and extracellular matrix.
When glycation affects the respiratory epithelium, it can lead to several harmful outcomes:
Barrier Integrity Loss: Glycated proteins in the epithelial cells compromise cell-cell junctions, weakening the tight junctions that hold the cells together. This loss of barrier integrity allows allergens, pathogens, and toxins to penetrate deeper into the lung tissue, triggering an immune response and causing inflammation.
Mucus Production and Ciliary Dysfunction: Glycation can also disrupt the normal function of cilia—tiny hair-like structures that help clear mucus and trapped particles from the respiratory tract. This disruption can lead to decreased mucociliary clearance, allowing mucus to accumulate, which further promotes a pro-inflammatory environment and increases the risk of respiratory infections.
Chronic Inflammation and Fibrosis: The accumulation of advanced glycation end-products (AGEs) in the respiratory epithelium can stimulate the release of pro-inflammatory cytokines, such as TNF-α and IL-6. This creates a chronic inflammatory state that may contribute to diseases like chronic obstructive pulmonary disease (COPD) and asthma. Over time, chronic inflammation can lead to fibrosis, or scarring of the lung tissue, which further impairs respiratory function.
2. Immune System Dysfunction and Dysregulation Leading to Inflammation
Glycation also significantly impacts the immune system, leading to its dysfunction and dysregulation. The immune system relies on the proper functioning of various cells, such as neutrophils, macrophages, and lymphocytes, to detect and respond to infections and other threats. Glycation can interfere with these cells’ normal activities in several ways:
Impaired Phagocytosis: Glycated proteins can reduce the ability of immune cells like macrophages and neutrophils to engulf and destroy pathogens (a process known as phagocytosis). This impairment weakens the body’s primary defense mechanism against infections, allowing pathogens to proliferate and causing prolonged or excessive inflammatory responses.
Altered Cytokine Release: Glycation can disrupt normal cell signaling by modifying proteins involved in cytokine production and release. As a result, immune cells may release excessive pro-inflammatory cytokines, such as IL-1, IL-6, and TNF-α, leading to a state of chronic, low-grade inflammation. This dysregulated cytokine release contributes to systemic inflammation and has been implicated in conditions such as rheumatoid arthritis, atherosclerosis, and even diabetes complications.
Autoimmunity and Immune Tolerance Breakdown: Glycated molecules can act as neo-antigens, which are recognized as foreign by the immune system. This recognition can trigger an autoimmune response, where the body’s immune system mistakenly attacks its tissues, contributing to autoimmune diseases like lupus or type 1 diabetes.
How to prevent further glycation?
Preventing further glycation involves several strategies that aim to control blood glucose levels, reduce oxidative stress, and minimize the formation of advanced glycation end-products (AGEs). Here are key approaches to help prevent glycation:
1. Maintain Healthy Blood Glucose Levels
Dietary Management: Follow a balanced diet that is low in refined sugars and carbohydrates to avoid spikes in blood glucose levels. Opt for foods with a low glycemic index, such as whole grains, legumes, vegetables, and lean proteins.
Regular Monitoring: Frequently monitor blood glucose levels, especially for individuals with diabetes, to ensure they remain within a healthy range. Fortunately continuous glucose monitors are now available over the counter. These are excellent to provide you with data about how your body processes various foods and the resulting spike.
Medications: Use prescribed medications like insulin or oral hypoglycemic agents as directed by a healthcare provider to manage blood glucose levels effectively. Similarly if your doctor has asked you to take anti-inflammatory meds, take them. These medicines will help reduce the cognitive decline that occurs due to peripheral/systemic inflammation.
2. Increase Physical Activity
Regular Exercise: Engage in regular physical activity, such as aerobic exercises, resistance training, or even daily walking, to help improve insulin sensitivity and lower blood glucose levels.
Incorporate Strength Training: Resistance exercises can enhance muscle mass, which helps improve glucose uptake and utilization by muscles, reducing excess glucose available for glycation.
3. Adopt Antioxidant-Rich Diet
Consume Antioxidant-Rich Foods: Include foods rich in antioxidants, such as fruits, vegetables, nuts, seeds, and green tea, to help neutralize free radicals that contribute to glycation and the formation of AGEs.
Supplements: Consider antioxidant supplements like vitamin C, vitamin E, alpha-lipoic acid, or N-acetylcysteine after consulting with a healthcare professional.
4. Limit Consumption of Processed Foods
Avoid High-AGE Foods: Reduce intake of foods that are high in AGEs, such as fried, grilled, roasted, or broiled meats. Cooking methods like steaming, boiling, or poaching produce fewer AGEs.
Minimize Processed Foods: Limit consumption of processed and packaged foods that often contain high amounts of sugars and additives, which can increase glycation.
5. Enhance Glyoxalase System Activity
Nutrient Support: Ensure adequate intake of nutrients that support the glyoxalase system, such as magnesium, zinc, and vitamin B6, which help detoxify methylglyoxal, a precursor to AGEs.
6. Stay Hydrated
Adequate Hydration: Drink plenty of water to support kidney function, which helps in the excretion of glucose and AGEs from the body, reducing their accumulation.
7. Consider Medications or Supplements to Inhibit AGE Formation
Pharmaceutical Interventions: Certain medications, such as metformin and ACE inhibitors, may help reduce AGE formation and accumulation.
Natural Compounds: Some natural compounds, such as aminoguanidine, benfotiamine, and carnosine, have been shown to inhibit AGE formation. Consult with a healthcare provider before starting any new supplements.
8. Reduce Smoking and Alcohol Consumption
Smoking Cessation: Quit smoking, as tobacco smoke contains reactive glycation products that can accelerate AGE formation.
Limit Alcohol: Reduce alcohol intake, particularly of beverages high in sugars, as excessive alcohol consumption can lead to spikes in blood glucose levels.
9. Manage Stress
Stress Management Techniques: Practice stress reduction techniques, such as mindfulness, meditation, yoga, or deep breathing exercises, as chronic stress can raise blood glucose levels and increase the risk of glycation.
10. Regular Health Check-Ups
Routine Monitoring: Regularly visit healthcare providers for monitoring and management of blood glucose levels, kidney function, and overall health, which can help detect and address any glycation-related complications early.
Can we remove existing glycation?
Removing existing glycation, particularly advanced glycation end-products (AGEs), is more challenging than preventing it, but certain strategies can help reduce the accumulation of these harmful compounds. These strategies include stimulating autophagy, adopting beneficial lifestyle changes, and utilizing specific supplements and treatments. Here are some of the most effective approaches:
1. Stimulate Autophagy
Intermittent Fasting: Practices like intermittent fasting (e.g., 16:8, 5:2) or prolonged fasting can stimulate autophagy, the body’s natural process of removing damaged cells, including those with glycated proteins. Autophagy helps to clear out old, dysfunctional cells and supports cellular renewal.
Caloric Restriction: Reducing overall caloric intake, particularly from carbohydrates and sugars, has been shown to promote autophagy and decrease AGE levels. Caloric restriction also reduces oxidative stress and inflammation, which can further mitigate the impact of glycation.
2. Adopt a Healthy Lifestyle
Regular Physical Activity: Consistent exercise can help reduce glycated proteins by improving insulin sensitivity, enhancing glucose metabolism, and promoting autophagy. Both aerobic exercise (like walking, running, cycling) and resistance training (like weight lifting) have been shown to be beneficial.
Adequate Sleep: Ensuring adequate, high-quality sleep is crucial, as poor sleep is associated with increased oxidative stress and impaired glucose metabolism, both of which can exacerbate glycation.
3. Use Targeted Supplements and Natural Compounds
Carnosine: Carnosine is a natural dipeptide that has been shown to inhibit AGE formation and break down existing glycated proteins. It also has antioxidant properties that protect cells from damage caused by AGEs.
Benfotiamine: A derivative of vitamin B1 (thiamine), benfotiamine has been shown to reduce AGE accumulation and protect against glycation-induced cellular damage, particularly in diabetic patients.
Alpha-Lipoic Acid (ALA): ALA is a potent antioxidant that can help reduce oxidative stress and may enhance the body’s ability to repair glycated tissues. It also supports glucose metabolism and insulin sensitivity.
N-Acetyl Cysteine (NAC): NAC is a precursor to glutathione, the body’s primary antioxidant, and may help reduce glycation by lowering oxidative stress and supporting cellular repair processes.
4. Enhance Cellular Repair and Regeneration
Diet Rich in Polyphenols and Flavonoids: Consuming foods rich in polyphenols and flavonoids, such as berries, green tea, dark chocolate, and certain spices (like turmeric and cinnamon), can support cellular repair mechanisms and have been shown to inhibit AGE formation.
Omega-3 Fatty Acids: Omega-3s, found in fatty fish, flaxseeds, and walnuts, have anti-inflammatory properties that may help reduce the inflammatory response caused by glycation and support cellular repair.
5. Utilize Medical Interventions
Dialysis or Plasma Exchange: In severe cases, such as in advanced diabetic nephropathy or systemic AGE accumulation, dialysis or therapeutic plasma exchange may help remove circulating AGEs from the blood.
Pharmaceutical Interventions: Certain medications, like metformin, ACE inhibitors, or ARBs, have shown potential in reducing AGE formation and accumulation, particularly in patients with diabetes.
6. Support Detoxification Pathways
Hydration: Drinking plenty of water aids kidney function and helps flush out excess AGEs through urine. Proper hydration is essential for efficient excretion of waste products, including AGEs.
Fiber-Rich Diet: Consuming a diet high in fiber supports digestive health and aids in the elimination of toxins, including AGEs, through regular bowel movements.
7. Reduce Exposure to Exogenous AGEs
Cook Using Low-Temperature Methods: Avoid cooking methods that produce high levels of AGEs, such as grilling, frying, and roasting. Opt for boiling, steaming, or poaching, which generate fewer AGEs.
Minimize Processed Foods: Avoid processed and packaged foods high in sugars, additives, and AGEs.
8. Enhance Mitochondrial Health
Support Mitochondrial Function: Maintain mitochondrial health with nutrients like Coenzyme Q10 (CoQ10), magnesium, and B vitamins to optimize cellular energy production and repair processes, potentially reducing the impact of glycation.
References
An overview on glycation: molecular mechanisms, impact on proteins, pathogenesis, and inhibition | Biophysical Reviews
https://link.springer.com/article/10.1007/s12551-024-01188-4
Recent advances in the epithelial barrier theory | International Immunology | Oxford Academic
https://academic.oup.com/intimm/article/36/5/211/7560334
Carnosine and advanced glycation end products: a systematic review – PubMed
https://pubmed.ncbi.nlm.nih.gov/29858687
Benfotiamine – an overview | ScienceDirect Topics
https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/benfotiamine
Taurine, alpha lipoic acid and vitamin B6 ameliorate the reduced developmental competence of immature mouse oocytes exposed to methylglyoxal | Scientific Reports
https://www.nature.com/articles/s41598-024-66785-5
Organosulfurs, S-allyl cysteine and N-acetyl cysteine sequester di-carbonyls and reduces carbonyl stress in HT22 cells | Scientific Reports
https://www.nature.com/articles/s41598-023-40291-6
DrBeen: Online Personalized Medical Education – DrBeen
What is Dementia? Symptoms, Causes & Treatment | alz.org
https://www.alz.org/alzheimers-dementia/what-is-dementia
doi:10.1016/j.cub.2008.04.047
https://www.cell.com/current-biology/pdf/S0960-9822(08)00533-2.pdf
Microglia – Wikipedia
https://en.wikipedia.org/wiki/Microglia
Systemic inflammation during midlife and cognitive change over 20 years – PMC
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6511107
Co-morbidity and systemic inflammation as drivers of cognitive decline: new experimental models adopting a broader paradigm in dementia research – PMC
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4369837
Systemic inflammation and disease progression in Alzheimer disease – PMC
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2848584
hsCRP, LDL, Lp(a)
Simple blood test could predict a person’s heart disease risk 30 years out, study finds
https://www.yahoo.com/news/simple-blood-test-could-predict-110000497.html
Inflammation, Cholesterol, Lipoprotein(a), and 30-Year Cardiovascular Outcomes in Women | New England Journal of Medicine
https://www.nejm.org/doi/full/10.1056/NEJMoa2405182
Lipoprotein(a): A Genetically Determined, Causal, and Prevalent Risk Factor for Atherosclerotic Cardiovascular Disease: A Scientific Statement From the American Heart Association | Arteriosclerosis, Thrombosis, and Vascular Biology
https://www.ahajournals.org/doi/10.1161/ATV.0000000000000147






I have been low carb, paleo but with dairy, for 8 yrs. 5 yrs ago fasting glucose was 4mmol/L and insulin was 2 mIU/L. HbA1c was 5.3 & 5.
Recent HbA1c is 5.7% so I bought a keto-mojo meter and can see my glucose readings are higher than I’d like and appropriate for that level of 5.7%. Where am I going wrong? I’ve heard of ‘physiological insulin resistance’ on low carb diets but I have too much glucose sloshing around in my blood and don’t know how to lower it.
My theories so far…..I’ve put on weight just due to overeating ( subcutaneous) so have to lose it to correct glucose.
Have I eaten too much protein in an effort to try and build muscle ( along with resistance exercise)?
I’ve had to double my progesterone part of my HRT and now my body thinks it’s pregnant and is insulin resistant ?
I’ve started fasting each week, between 1-3 days and after about 24 hrs my glucose levels come down to a better level. I managed ketones of 1 at 1 day, 2-3 on day 2 and 3-4 on 3rd day.
Why isn’t being low carb enough?
Thanks!
Was reading this excellent substack paper and listening to CNBC. One of the panelist mention an FDA approved AI assisted diagnosis of diabetic retinopathy. Missed the company name but search found this:
https://www.hcplive.com/view/fda-authorizes-autonomous-ai-portable-diabetic-retinopathy-screening
Cute name: AEYE.